Energy - 1.2.1 Energy Transfers in a System (GCSE Physics AQA)
Energy Transfers in a System
Conservation of Energy
The conservation of energy is an important principle in Physics. According to this principle, we can’t ‘lose’ or ‘gain’ energy:
Energy can be transferred usefully, stored or dissipated, but cannot be created or destroyed.
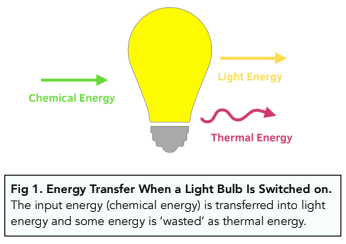
Since we know that energy cannot be created or destroyed, all the energy from a system must be dissipated somehow. The dissipated energy can be useful, or not useful (e.g. wasted energy).
In many energy transfers, thermal energy is a waste product. For example, when we switch on a light bulb, chemical energy is being transferred to light energy, but some of the energy will be ‘wasted’ as thermal energy.
Reducing Unwanted Energy Transfers
There are several ways in which we can reduce the amount of unwanted energy transfers:
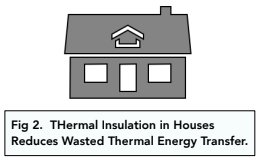
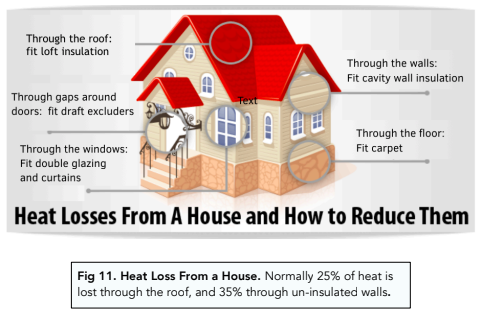
- Thermal insulation. A common way in which energy is wasted is through thermal energy. For example, when a house is heated, energy can be transferred through the windows, doors and walls. In order to combat this, houses are often insulated and windows are double glazed.
Energy Transfers in a Closed System
- Closed systems don’t exchange with their surroundings. In section 4.1.1.1, we mentioned that closed systems are unable to exchange energy to matter with their surroundings. For example, a thermos flask is a closed system as heat cannot escape (ignoring negligible amounts of heat loss)
- Energy transfers can occur in closed systems. Like any other system, energy can be transferred in a close system. However, since energy cannot exchange with the surroundings, there will be no net change to the total energy in a closed system.
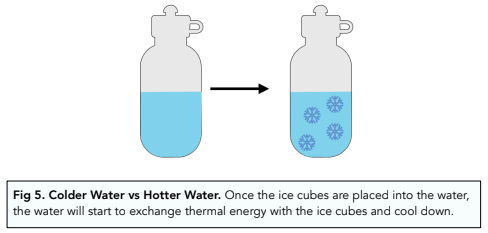
- Adding ice cubes to a water bottle is an energy transfer. If you put ice cubes into a full water bottle and close the lid, you are transferring energy. We are assuming that the water bottle doesn’t allow any energy exchange with the surroundings, creating a closed system. The water will exchange thermal energy with the ice cubes, so the water will cool down.
Thermal Conductivity
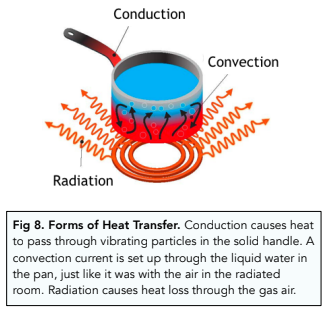
- Thermal energy is transferable. Thermal energy can be transferred between objects, or from an object to its surroundings. For example, when we heat up soup in a saucepan, thermal energy will be transferred from the pan to the soup, then from the soup to the surroundings.
- The rate of transfer depends on thermal conductivity. Thermal conductivity will determine how fast the thermal energy is transferred. For AQA exams, you don’t need to know the definition of thermal conductivity, but you should know how it works. During conduction, vibrating particles transfer heat energy to adjacent particles, and the vibration passes along, leading to the spread of heat. The higher the thermal conductivity of an object, the higher the rate of thermal energy transfer
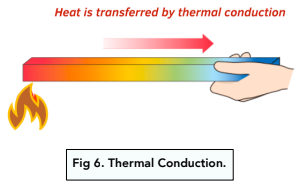
- Conduction is primarily in solids. Conduction is the main form of heat transfer in solids. This is because the particles are close by and can pass on vibrations easily, which is harder in a gas. Heat in liquids and gases is normally transferred by convection and radiation, which we will touch on below.
Conduction, Convection and Radiation
Heat is usually transferred by conduction in solids. In liquids and gasses, convection and radiation are more important. You don’t need to know them in detail for AQA, so here is a brief overview:
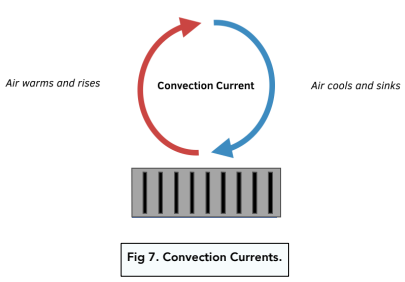
- Convection – radiators transfer heat by convection. Air near the radiator is heated, which causes it to become less dense. Therefore this warm, less dense air rises up the room, and the cold, denser air above it sinks down. The cold air can now be heated by the radiator. This cycle goes on and on, and a convection current is formed. The heat is spreading through the room via this current.
- Radiation – radiation involves transfer of heat via infra red waves. The sun provides heat to the earth due to radiation.
Cooling a Building
When we heat up our homes, we want them to stay warm for as long as possible. However, the thermal conductivity of the doors, windows and walls can result in energy losses. In order to lessen the amount of thermal energy that escapes from our homes, we need to reduce thermal conductivity. This can be achieved in the following ways:
- Double glazed windows – by using double glazing, we are thickening the windows and therefore reducing their thermal conductivity. As the thermal conductivity decreases, the rate of thermal energy transfer will also decrease.
Investigating Thermal Insulators
Method
- Gather the equipment. For this experiment, you will need a beaker (with a lid) filled with hot water, a thermometer, a top pan balance, a stopwatch and different insulating materials.
- Measure the filled beaker’s mass. Place the beaker full of hot water onto a top pan balance. Make sure that the lid is on top of the beaker too. Record the mass in kilograms.
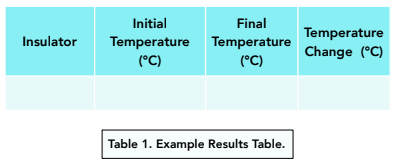
- Measure the water temperature. Use the thermometer to measure the temperature of the water in the beaker. Record this as the ‘initial temperature’, with the units in °C.
- Time for 5 minutes. Make sure that you have placed the lid on the beaker. Start the stopwatch and time for five minutes.
- Measure the water temperature. Once the five minutes are up, you can measure the temperature of the water again. Recorded this as the ‘final temperature’, with the units in °C.
- Calculate the temperature change. To find the change in temperature, do the calculation ‘initial temperature – final temperature’. Record this as the ‘temperature change’, with the units in °C.
- Repeat steps 2-6 with different insulators. For each experiment, make sure that you have the same mass of water and the same initial temperature. The only thing that should change is the material that you wrap around the beaker.
- Record your results in a table. The results table should look like this:
Energy transfer is the movement of energy from one place or object to another. Energy can be transferred in many different ways, including through heat, light, sound, and movement.
In physics, a system refers to an object or group of objects that are being studied and analyzed. In the context of energy transfers, a system is defined as the specific object or objects that are undergoing an energy transfer.
There are three main types of energy transfers in a system: thermal (heat), electrical, and mechanical (movement).
Thermal energy transfer, also known as heat transfer, occurs when energy is transferred from one object to another as a result of a temperature difference between the two objects. This type of energy transfer is what makes hot objects feel hot to the touch, and it can occur through conduction, convection, or radiation.
Electrical energy transfer occurs when energy is transferred from one place to another through the flow of electrons. This type of energy transfer is what powers most of the electrical devices we use in our daily lives, and it can be conducted through wires, conductive materials, or even through the air.
Mechanical energy transfer occurs when energy is transferred from one object to another as a result of movement. This type of energy transfer is what allows us to do work, such as lifting a heavy object, and it can be transferred through direct contact or through the transfer of energy through a medium, such as a rope or chain.
Energy transfers in a system occur as a result of interactions between the objects in the system. Energy can be transferred through the movement of matter, through the transfer of heat or light, or through the flow of electrons. The specific type of energy transfer that occurs in a system depends on the interactions between the objects and the energy states of those objects.
Understanding energy transfers is important in GCSE Physics AQA because it is a fundamental concept in the study of physics. Understanding how energy transfers occur and the factors that affect the transfer of energy is key to understanding many other concepts in physics, such as thermodynamics, electricity, and mechanics.






Still got a question? Leave a comment
Leave a comment