Quantum Calculations
Summary of Quantum Theory
The utilization of quantum theory is employed to elucidate the relationships between matter and energy at the minuscule level of atoms and subatomic particles.
Basic principles:
- Photons are the elementary units in which energy is quantized
- Elementary particles display the characteristics of both particles and waves
- The movement of particles is stochastic in nature
- There exists an inherent uncertainty in determining the position and momentum of particles simultaneously
Planck’s Quanta
The notion that light was discharged in distinct bundles of energy known as quanta was first proposed by Max Planck in 1901. Planck’s equation describes the energy of each quantum as
E = hν
where,
E = represents energy in Joules (J)
h = is Planck’s constant, which is equivalent to 6.626 x 10-34 Js
ν(nu) = is the frequency of light radiation (Hz, s-1)As part of his photoelectric effect theory, Einstein referred to these quanta as streams of particles known as photons.
Wave-particle duality
Mistakenly, particles are sometimes considered as ‘matter waves’ or ‘energy waves’ (such as photons).
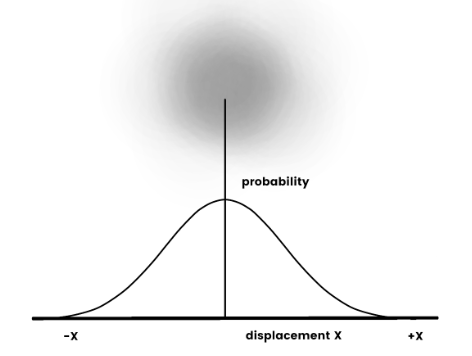
The wave properties of particles relate to their statistical position around a point. Rather than behaving like a solid object, a particle can be likened to a fuzzy ball, where the intensity of color denotes position. Thus, the particle remains primarily in the center of fuzziness and least at the edges.
The Copenhagen interpretation serves as a clarification on the issue of the reality of particle duality.
The statement implies that the existence of anything is dependent on its observation. Hence, a material point can be viewed either as a particle or a wave. If its behavior is akin to that of a particle, then it is indeed a particle. Conversely, if its behavior mimics that of a wave, then it is a wave
Uncertainty Principle of Heisenburg
For a particle, position and momentum cannot be known at the same time.
Mathematically the product of the uncertainty in the position of a particle (Δx)and the uncertainty in the momentum (Δp) could never be less than a particular value. That particular value is related to Planck’s constant h
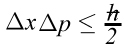
The Wave Function
The Schrödinger Wave Equation offers a comprehensive account of the wave-like properties of matter. It is utilized to forecast the future behavior of a system in motion. It is important to note that the waves mentioned in the equation refer to ‘probability waves’.
Shown below is the Schrödinger wave equation for a single-direction, free particle. The wave function in this equation is represented by the symbol Ψ.
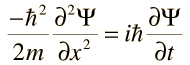
The wave function provides information on:
- Probability distributions across one, two, or three dimensions
- Quantum states
- Energy levels
There are various iterations of the wave equation to accommodate different scenarios.
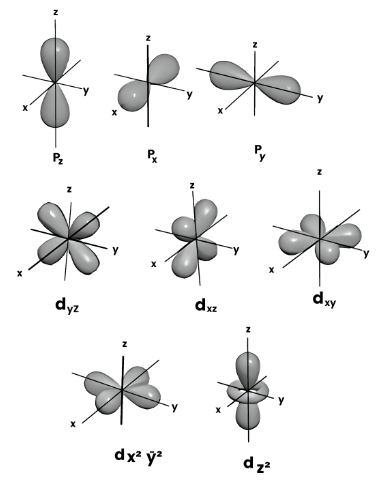
One of the equations forecasts the location of electrons in the vicinity of an atomic nucleus. Electrons are distributed in discrete three-dimensional zones known as ‘orbitals’. This research was predominantly undertaken by Wolfgang Ernst Pauli, who introduced the famous ‘exclusion principle’.
Quantum Tunneling
This is a phenomenon that concerns the likelihood wave of an electron and its spread across an insurmountable obstruction.
In classical terms, the electron lacks the necessary kinetic energy to surmount a potential energy obstruction. It is comparable to a bouncing ball that cannot leap over a high brick wall.
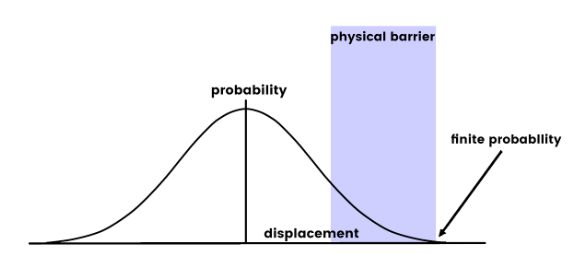
Electrons are matter particles that exhibit wavelike characteristics. As per the principles of quantum mechanics, the wave function (likelihood wave) of the electron spreads out beyond the barrier.
Consequently, there is a finite chance of its existence beyond the barrier.
Quantum tunneling is utilized in quantum tunneling composites (QTC’s) and tunneling diodes.
Tunneling diodes are critical components in high-frequency devices, such as frequency generators, detectors, and CROs.
‘Quantum tunneling composites’ refers to the technology used in ‘touch screens.’ They have numerous applications, including phones, TVs, cameras, and monitors.
Quantum Entanglement
Even if separated across vast distances, quantumly entangled particles maintain a connection, so any disturbance to one particle will also affect the other.
The phenomenon was famously described by Einstein as “spooky action at a distance”, reflecting his skepticism towards it.
For instance, when two electrons interact and become entangled, they share a state that correlates their properties.
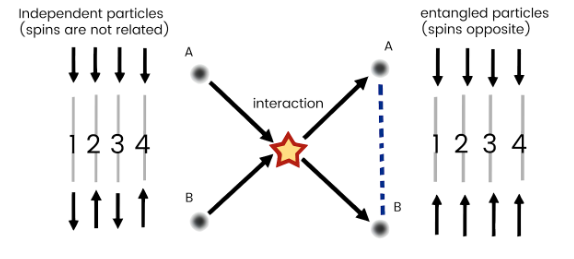
In an experiment, each number in the diagram represents a run where the spins of particles A and B are measured.
As a result of entanglement, the spin states of the two particles become complementary, with one being ‘up’ while the other is ‘down’.
This shared state allows for any change to one electron to be instantly reflected in the other, offering enormous potential for the development of quantum computers, which could greatly outpace current computing technology.
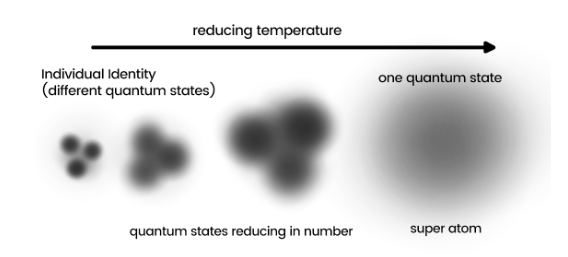
Bose-Einstein Condensate (BEC)
When the temperature approaches absolute zero, the lowest energy level of gas atoms is reached, and quantum mechanical effects become more pronounced.
Due to the uncertainty of their positions, atoms can be visualized as blurry balls. As temperature decreases, these balls expand and become less well-defined. Eventually, they begin to overlap, leading to a point where their fuzziness (position) merges.
At this juncture, the atoms lose their individual identities and merge into a single “super atom” with the same quantum state.
Quantum calculations involve the application of mathematical concepts and principles to analyze and predict the behavior of subatomic particles and systems. In A-Level Physics, you will learn about the basics of quantum mechanics, wave-particle duality, quantum numbers, and quantum states, which form the basis for quantum calculations.
Quantum mechanics is a fundamental theory of physics that governs the behavior of matter and energy at the subatomic level. Understanding quantum mechanics is crucial for A-Level Physics students as it explains many physical phenomena and has practical applications in areas such as electronics, materials science, and quantum computing.
Some common topics covered in quantum calculations include the Schrödinger equation, wave functions, probability densities, quantum tunneling, quantum entanglement, and the uncertainty principle.
To improve your understanding of quantum calculations, you should start by mastering the mathematical concepts involved, such as linear algebra and differential equations. You can also practice solving problems and doing exercises to reinforce your knowledge. Finally, seeking help from your teachers, classmates, or online resources can be beneficial.
Quantum calculations have many practical applications, such as in the development of new materials with unique properties, the design of more efficient electronic devices, and the creation of quantum computers, which could revolutionize computing power and encryption methods.
Yes, quantum calculations can be challenging and computationally expensive due to the complexity of the quantum systems being analyzed. Moreover, the probabilistic nature of quantum mechanics means that the results of calculations can be uncertain and subject to interpretation.
To prepare for exams on quantum calculations, you should review your notes and textbook, do practice problems and past papers, and seek feedback from your teachers or tutors. You should also make sure to understand the underlying principles and concepts, rather than just memorizing formulas and equations.





Still got a question? Leave a comment
Leave a comment